Density Cubes
It turns out you can buy little cubes of various elements from sketchy Amazon marketplace vendors.
Naturally, I wanted some Tungsten, because of its incredible density.
A few months ago, I got what were billed as tungsten and aluminum cubes. The nominally tungsten cube was attracted to magnets.
Recently, I got a five cube set of tungsten, niobium, titanium, nickel and copper, and a single cobalt cube. I wanted to verify that nickel and cobalt were ferromagnetic. This second (putatively) tungsten cube was non-magnetic. I’d also like to verify that I got niobium, nickel, cobalt and titanium.
Materials
Metal cubes
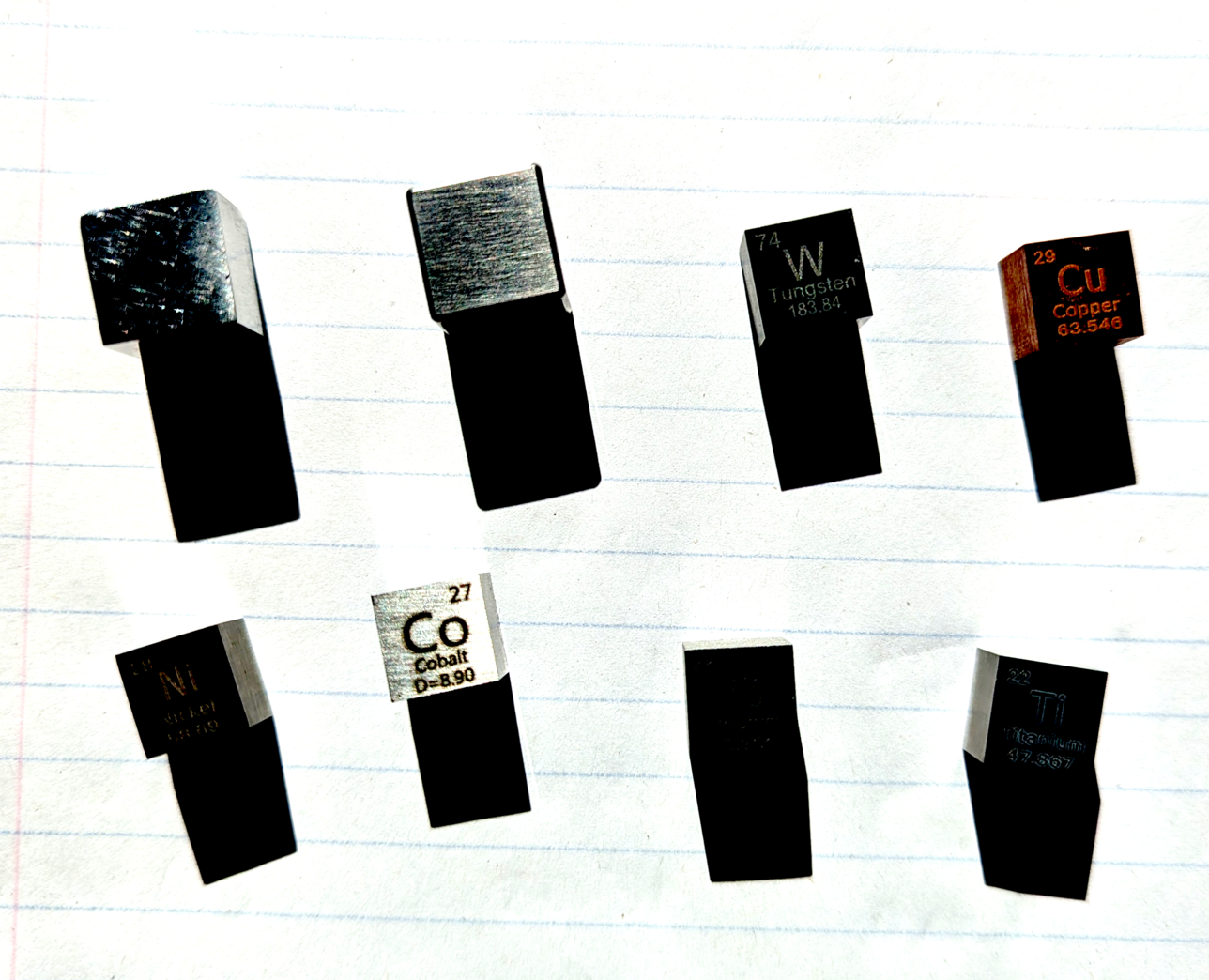
Eight cubes of various metals. Top row: aluminum, tungsten (A), tungsten (B), copper. Bottom row: nickel, cobalt, niobium, titanium. I ran the saturation and exposure up on the above image so the text stenciled on some of the cubes is visible.
Scale

An AWS-100 scale, supposedly accurate to 0.01 gram.
Micrometer

A GeneralTM brand micrometer, said to be accurate to 0.001 inch. I think I bought it at Lowe’s or Home depot.
Dial Calipers
Smiec brand calipers, supposedly accurate to 0.001 inch. I got this years ago, from Edmond Scientific, some time before 1989.
Procedure
- Weight the cubes with the AWS-100.
- Measure the cubes with micrometer.
I put the micrometer’s cylinders in the middle of the cubes’ faces.
Six of the cubes have some periodic table data stenciled on one face,
so I was able to measure:
- with the text
- across the text
- normal to the text.
The other two cubes, which are larger, I held in one orientation and carefully measured the 3 axes. This micrometer has a slip-clutch on the end you rotate, I twisted the clutch handle until it slipped, then read the scale.
- Measure the cubes with the calipers. The caliper has a crumby friction wheel you work with your thumb. It never slipped, so all the measurements are with a firm caliper squeeze. I measured with the cubes in the shaved-down tip portion of the caliper jaws, measuring all three axes of the cubes as with the micrometer.
Results
| Putative material | mass | volume 1 | volume 2 | measured density | nominal density |
|---|---|---|---|---|---|
| tungsten (A) | 36.85 | 2.07 | 2.05 | 17.89 | 19.254 |
| aluminum | 5.51 | 2.07 | 2.05 | 2.67 | 2.71 (1100 grade) |
| tungsten (B) | 20.31 | 1.07 | 1.05 | 19.16 | 19.254 |
| copper | 8.97 | 1.03 | 1.02 | 8.75 | 8.935 |
| nickel | 8.98 | 1.03 | 1.02 | 8.76 | 8.907 |
| cobalt | 8.85 | 1.02 | 1.00 | 8.76 | 8.834 |
| niobium | 8.67 | 1.02 | 1.02 | 8.50 | 8.582 |
| titanium | 4.56 | 1.03 | 1.03 | 4.43 | 4.502 |
- Putative tungsten (A) is magnetic
- Putative tungsten (B) is non-magnetic
- Mass measured in grams.
- Volume 1 is measured with micrometer, cm3.
- Volume 2 is measured with caliper, cm3.
- Measured density is 2*mass/(volume 1 + volume 2), grams per cubic centimeter.
Conclusions
My measured density is low across the board. I assumed a cubic shape. Maybe the pieces of metal are far enough away from cubes to matter. Or I may have some systematic error when measuring, or my scale may weigh slightly low.
The aluminum, niobium, copper and titanium seem like they are correctly portrayed. That surprises me, since niobium and titanium aren’t everyday materials.
The putative nickel cube is the same density as the putative cobalt cube. They’re both magnetic. Iron (and mild steel) has a density of 7.874 g/cm3. I don’t think they’re iron or steel, I’m not measuring density that far off. These two cubes don’t have any rust, either. I may have 2 nickel cubes.
The tungsten cubes are the hardest to make sense of. Tungsten cube (B) is a little less dense than nominal tungsten, I’m going to call it tungsten, or tungsten with only a little alloy. Cube (B) is utterly non-magnetic.
Tungsten cube (A) is a lot less dense than nominal tungsten, but somewhat denser than tungsten Carbide, which has a nominal density of 15.6 g/cm3 Cube (A) will stick to a magnet. I may have a cube made of sintered tungsten carbide power with nickel or cobalt holding the tungsten carbide grains together. Whether or not real tungsten carbide is magnetic is harder to ascertain. This cube’s material is still indeterminate.
